Immunoglobulin M (IgM) is one of the five types of immunoglobulins in the human body, playing an important role in the early stages of the immune response. IgM can exist in several distinct forms, including monomeric, membrane-bound IgM within the B cell receptor (BCR) complex, pentameric and hexameric IgM in serum and secretory IgM (sIgM) on the mucosal surface1.In previous work, Junyu Xiao’s research group elucidated the molecular mechanism of IgM pentamer assembly and mucosal transport, revealing that IgM forms pentamers in an asymmetric manner and the structural basis of J chain regulation of IgM pentamer assembly, which mediates its interaction with the mucosal transport receptor pIgR.

Fc receptor is one important type of receptors in the human immune system, which recognizes Fc region of immunoglobulins to elicit distinct effector mechanisms. Fc receptors play a crucial role in regulating immune response and mediating cell toxicity. Different immunoglobulins utilize different Fc receptors, which lead to the specificity of different signal pathways and immune responses. FcμR (also known as Toso or Faim3) is the only IgM-specific receptor in mammals, mainly expressed on the surface of B cells, and also expressed on other immune cells such as T cells. FcμR interacts with all forms of IgM, including pentameric and hexameric IgM, as well as mIgM within the BCR, thus participating in B cell development, immune homeostasis and antigen presentation 2-5. The high expression of FcμR on the surface of B cells in patients with chronic lymphocytic leukemia also highlights its importance in the immune system and disease occurrence 2,6-9. However, the molecular mechanism of FcμR function is not yet clear.
On March 22, 2023, Junyu Xiao’s research group published a study entitled "Immunoglobulin M Perception by FcμR" in Nature, revealing the molecular mechanism by which FcμR recognizes different forms of IgM.
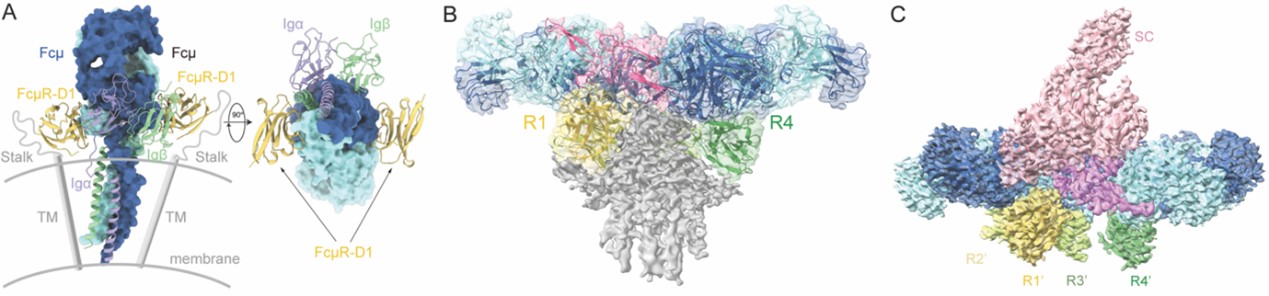
The extracellular domain (ECD) of FcμR contains an immunoglobulin-like domain (D1) followed by an intrinsically disordered, highly O-linked glycosylated stalk region. To investigate the recognition mechanism of FcμR for different forms of IgM, the study first expressed and purified the FcμR-D1 domain and IgM-Cμ4 domain complex and determined its crystal structure. The results showed that two FcμR-D1 domains bind to the two sides of an IgM-Cμ4 dimer. Comparing with the recently solved IgM-BCR complex structures10-12, the presence of FcμR did not affect the binding of IgM to Igα/Igβ (CD79α/CD79β), consistent with previous studies3,4. The study then used cryo-EM microscopy to determine the structure of the complex of the core region of IgM pentamer and FcμR-ECD. The results showed that FcμR can bind to the same side of the IgM pentamer in a ratio of 4:1, which suggests that the IgM pentamer may initiate downstream signaling by inducing the formation of FcμR tetramers. The stalk region may further mediate interaction between the four FcμR molecules, facilitating their binding to the same side of the IgM pentamer.
Interestingly, among four FcμR binding sites, the high-affinity R1 site overlaps with the binding site of the D1 domain of the secretory component (SC) in sIgM. Previous studies have shown that FcμR can bind to SC-containing sIgM and regulate its reverse transport on the mucosal side, thus participating in the antigen presentation5. How does FcμR bind to sIgM? To answer this question, the study further used cryo-EM microscopy to determine the structure of the complex of FcμR-ECD and the core region of sIgM. The results showed that when the SC is present, four FcμR molecules simultaneously bind to the other side of the IgM plane. It is worth mentioning that structural analysis showed that the J chain caused asymmetry on both sides of the IgM plane in different ways, so that IgM pentamer can only bind to a maximum of four FcμR molecules regardless of which side it is on. To evaluate the functional relevance of the above study, the study further designed FcμR mutants and verified the results by in vitro protein interaction, fluorescence confocal microscopy and flow cytometry.
In summary, this study reveals the molecular mechanism of FcμR's specific recognition of different forms of IgM through structural biology, biochemistry and cell biology methods, laying the foundation for a deeper understanding of the biological function of IgM.
Link to the article: https://www.nature.com/articles/s41586-023-05835-w.
1 Li, Y. et al. Structural insights into immunoglobulin M. Science 367, 1014-1017, doi:10.1126/science.aaz5425 (2020).
2 Kubagawa, H. et al. Identity of the elusive IgM Fc receptor (FcmuR) in humans. J Exp Med 206, 2779-2793, doi:10.1084/jem.20091107 (2009).
3 Ouchida, R. et al. FcmuR interacts and cooperates with the B cell receptor To promote B cell survival. J Immunol 194, 3096-3101, doi:10.4049/jimmunol.1402352 (2015).
4 Nguyen, T. T. et al. The IgM receptor FcmuR limits tonic BCR signaling by regulating expression of the IgM BCR. Nat Immunol 18, 321-333, doi:10.1038/ni.3677 (2017).
5 Rochereau, N. et al. Essential role of TOSO/FAIM3 in intestinal IgM reverse transcytosis. Cell Rep 37, 110006, doi:10.1016/j.celrep.2021.110006 (2021).
6 Pallasch, C. P. et al. Overexpression of TOSO in CLL is triggered by B-cell receptor signaling and associated with progressive disease. Blood 112, 4213-4219, doi:10.1182/blood-2008-05-157255 (2008).
7 Proto-Siqueira, R. et al. SAGE analysis demonstrates increased expression of TOSO contributing to Fas-mediated resistance in CLL. Blood 112, 394-397, doi:10.1182/blood-2007-11-124065 (2008).
8 Li, F. J. et al. Enhanced levels of both the membrane-bound and soluble forms of IgM Fc receptor (FcmuR) in patients with chronic lymphocytic leukemia. Blood 118, 4902-4909, doi:10.1182/blood-2011-04-350793 (2011).
9 Vire, B., David, A. & Wiestner, A. TOSO, the Fcμ receptor, is highly expressed on chronic lymphocytic leukemia B cells, internalizes upon IgM binding, shuttles to the lysosome, and is downregulated in response to TLR activation. The Journal of Immunology 187, 4040-4050 (2011).
10 Su, Q. et al. Cryo-EM structure of the human IgM B cell receptor. Science 377, 875-880, doi:10.1126/science.abo3923 (2022).
11 Ma, X. et al. Cryo-EM structures of two human B cell receptor isotypes. Science 377, 880-885, doi:10.1126/science.abo3828 (2022).
12 Dong, Y. et al. Structural principles of B cell antigen receptor assembly. Nature 612, 156-161, doi:10.1038/s41586-022-05412-7 (2022).